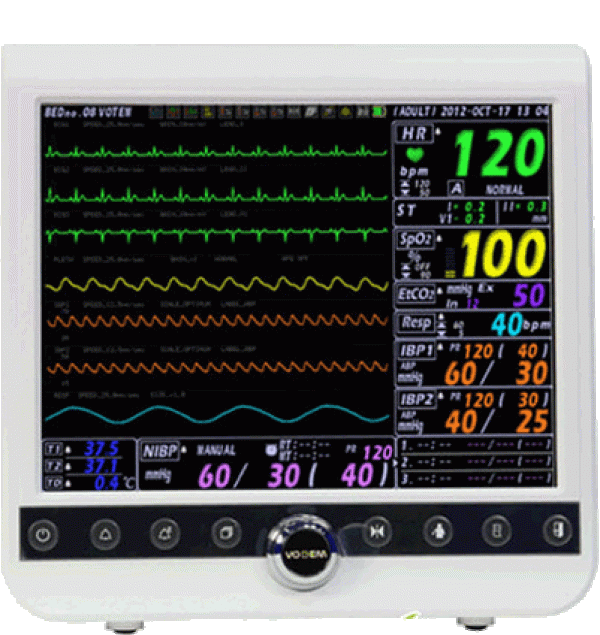
electrocardiogram (ECG)
Safety : IEC 60601-1 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
IEC 60601-2-25 Medical electrical equipment – Part 2-25: Particular requirements for the basic safety and essential performance of electrocardiographs IEC 80601-2-49:2018 Medical electrical equipment - Part 2-49: Particular requirements for the basic safety and essential performance of multifunction patient monitors
EMC : IEC 60601-1-2: 2014+AMD1:2020 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests CISPR 11:2015 Industrial, scientific and medical equipment - Radio-frequency disturbance characteristics - Limits and methods of measurement
Performance : IEC 60601-2-27:2011 Medical electrical equipment - Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment
IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests
Local Standard
USA :
ANSI/AAMI ES60601-1:2005/A2:2021 Medical Electrical Equipment - Part 1: General Requirements For Basic Safety And Essential Performance - Amendment 2
21CFR870.2300 MEDICAL DEVICES Subpart C - Cardiovascular Monitoring Devices
ANSI/AAMI/IEC 60601-1-2:2014 Medical Electrical Equipment - Part 1-2: General Requirements For Basic Safety And Essential Performance - Collateral Standard: Electromagnetic Disturbances - Requirements And Tests
ANSI/AAMI EC13-2002 Cardiac Monitors, Heart Rate Meters, And Alarms
ANSI/AAMI ES60601-1:2005 (R2012) with amendments Medical Electrical Equipment - Part 1: General Requirements For Basic Safety And Essential Performance (IEC 60601-1:2005, MOD)
ANSI/AAMI/IEC 60601-2-25:2011 (R2016) Medical Electrical Equipment - Part 2-25: Particular Requirements For The Basic Safety And Essential Performance Of Electrocardiographs
ANSI/AAMI/IEC 60601-2-27:2011 (R2016) Medical Electrical Equipment - Part 2-27: Particular Requirements For The Basic Safety And Essential Performance Of Electrocardiographic Monitoring Equipment
Laser Products. [1040.10]; Specific Purpose Laser Products. [1040.11];
Europe:
EN 60601-1:2006(MAIN) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
EN 60601-1-2:2015 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
EN 60601-2-25:2015(MAIN) Medical electrical equipment - Part 2-25: Particular requirements for the basic safety and essential performance of electrocardiographs
EN 60601-1-2:2015(MAIN) Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests
EN 60601-2-47:2015(MAIN) Medical electrical equipment - Part 2-47: Particular requirements for the basic safety and essential performance of ambulatory electrocardiographic systems
EN 60601-2-27:2014(MAIN) Medical electrical equipment - Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment
EN IEC 80601-2-49:2019(MAIN) Medical electrical equipment - Part 2-49: Particular requirements for the basic safety and essential performance of multifunction patient monitoring equipment
China:
GB 9706.1: General requirements for safety of medical electrical equipment
GB 4824.1: General requirements for electromagnetic compatibility of medical electrical equipment
GB 21556-2008 Medical electrical equipment - Particular requirements for the basic safety and essential performance of electrocardiographs
GB/T 31467.3-2015 Energy consumption label for electric medical devices - Part 3: Electrocardiographs
Japan:
JIS T 0601-1: General requirements for safety of medical electrical equipment
JIS T 0601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral Standard: Electromagnetic disturbances - Requirements and tests
JIS T 15001-2-49:2017: Medical electrical equipment - Part 2-49: Particular requirements for the basic safety and essential performance of multifunction patient monitoring equipment
JIS T 1501-2:2005 - Electrocardiographs - Part 2: Specific requirements for safety and performance
Korea:
KS IEC 60601-1:2012 - Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
KS C 9811 Industrial, scientific and medical equipment - Radio-frequency disturbance characteristics - Limits and methods of measurement KS C IEC 60601-1-2:2016 is Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
KS C IEC60601-1-2: Medical electrical equipment — Part 1-2: General requirements for basic safety and essential performance — Collateral standard: Electromagnetic disturbances — Requirements and tests
KS C IEC6001-2-25: Medical electrical equipment ─ Part 2-25: Particular requirements for basic safety and essential performance of electrocardiographs
KS C IEC60601-2-27: Medical electrical equipment ─ Part 2-27 : Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment
Taiwan:
CNS 60601:2015 Medical electrical equipment - Part 1: General requirements for safety
CNS 60601-1-2 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests
CNS 14592:2016 Electrocardiographs
Brasil:
ABNT NBR IEC 60601-1:2015 Medical Electrical Equipment - Part 1: General Requirements for Basic Safety and Essential Performance
ABNT NBR IEC 60601-1-2:2015 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
ABNT NBR16628 DE 09/2017 Disposable ECG electrodes - Requirements
Mexico:
NOM-IEC 60601-1:2014 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
NOM-IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
NOM-240-SSA1-2012 Goods and services. Medical equipment. Electrocardiographs. Safety and efficacy specifications and test methods
Argentina:
IRAM 60601-1:2012 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
IRAM 60601-1-2:2012 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
IRAM 4220-2-25 Electromedical devices. Part 2-25: Particular safety requirements for electrocardiographs.
IRAM 4220-2-49 Electromedical devices. Part 2-49 - Particular safety requirements for multifunction devices for patient monitoring.
IRAM 4220-2-27 Electromedical devices. Part 2-27: Particular safety requirements for electrocardiographic monitoring devices.
Australia:
AS/NZS IEC 60601.1:2015 Medical electrical equipment, Part 1: General requirements for basic safety and essential performance
AS IEC 60601.1.2:2017 Medical electrical equipment, Part 1.2: General requirements for basic safety and essential performance — Collateral Standard: Electromagnetic disturbances — Requirements and tests
AS/NZS IEC 60601.2.25:2016 Medical electrical equipment - Part 2.25: Particular requirements for the basic safety and essential performance of electrocardiographs
AS/NZS IEC 60601.2.27:2016 Medical electrical equipment, Part 2.27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment
South Africa:
SANS 60601-1:2016 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
SANS 60601-1-2:2022 (Ed. 4.01):Medical electrical equipment -Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests
Saudi Arabia:
SASO IEC 60601-1:2015 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
SASO IEC 60601-1-2:2015 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
GSO IEC 80601-2-49:2021 IEC 80601-2-49:2018 Medical Electrical Equipment - Part 2-49: Particular Requirements For The Basic Safety And Essential
EAEU:
GOST R IEC 60601-1:2017 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
GOST R IEC 60601-1-2:2017 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances - Requirements and tests
GOST R 56326-2017 Medical equipment. Multifunction patient monitoring equipment. Technical requirements for governmental purchases
GOST R 56326-2014 Medical electrical equipment. Multifunction patient monitoring equipment. Technical requirements for governmental purchases